Deep-brain neurostimulation, a procedure designed and tested in Grenoble in particular by Professor Benabid and practiced for 34 years, has significantly reduced motor symptoms of 200,000 people with Parkinson's disease worldwide. Clinatec, the Edmond J. Safra Biomedical Research Center and a CEA laboratory based in Grenoble, is working on a complementary practice through the NIR project.
NIR device implantation © CEA-Leti
Traditional deep-brain stimulation delivers a high-frequency electrical current to modulate neural targets, which is pathologic in Parkinson's disease. In contrast, near-infrared illumination targets the substantia nigra, the site of the degeneration of the dopaminergic neurons that cause the motor symptoms of Parkinson's disease. Unlike current therapies that temporarily limit symptoms without affecting the degeneration of neurons, light could potentially halt degeneration of these neurons. Thus, this therapeutic approach could slow down the loss of motor functions of patients and their autonomy.
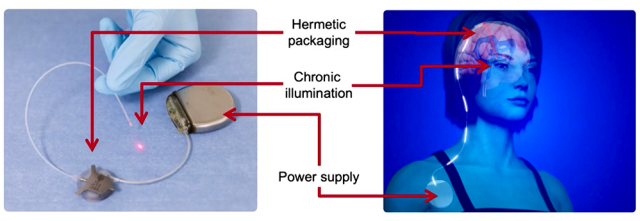
NIR photobiomodulation technology
The illumination is achieved using an implantable intracerebral device developed at CEA-Leti, a pioneer in the fields of micro- and nano-technology, in partnership with Boston Scientific. This system consists of an implantable neurostimulator, connected to an optical generator, itself connected to an optical fiber delivering near infrared light to the brain.
The potential of this approach was scientifically demonstrated in the aforementioned preclinical trial, the results of which were published, among others, in Annals of Neurology in 2016[1]. In view of these excellent results, the NIR project clinical trial was initiated in March 2021. The trial will follow patients for four years. It is the culmination of 10 years of research, both from a fundamental and technological development perspectives. CEA-Leti and Boston Scientific pooled their expertise in biology, experimental surgery, implanted medical devices, optics, and packaging to achieve this result.
As part of this clinical trial, Professor of Grenoble Alpes University Stephan Chabardès, neurosurgeon at Grenoble Alps University Hospital and medical director of Clinatec, successfully operated on the first patient, whose progress will be monitored by clinical evaluation but also by metabolic imaging performed in collaboration with the CERMEP imaging center in Lyon, which allows for the tracking of the effects of this therapeutic innovation.
The clinical protocol
The clinical trial team is seeking for 14 subjects. This research protocol is open to Parkinson's disease patients, under 65 years of age, who were diagnosed with Parkinson's disease less than 2 years ago, and who meet all inclusion criteria. Find all inclusion criteria
here.
Several hospitals have joined the Grenoble Alps University Hospital and Clinatec to collaborate in this clinical trial and thus facilitate the recruitment of these patients: the Hospices Civils de Lyon, the Assistance Publique des Hôpitaux de Marseille and the Henri Mondor Hospital in Créteil.